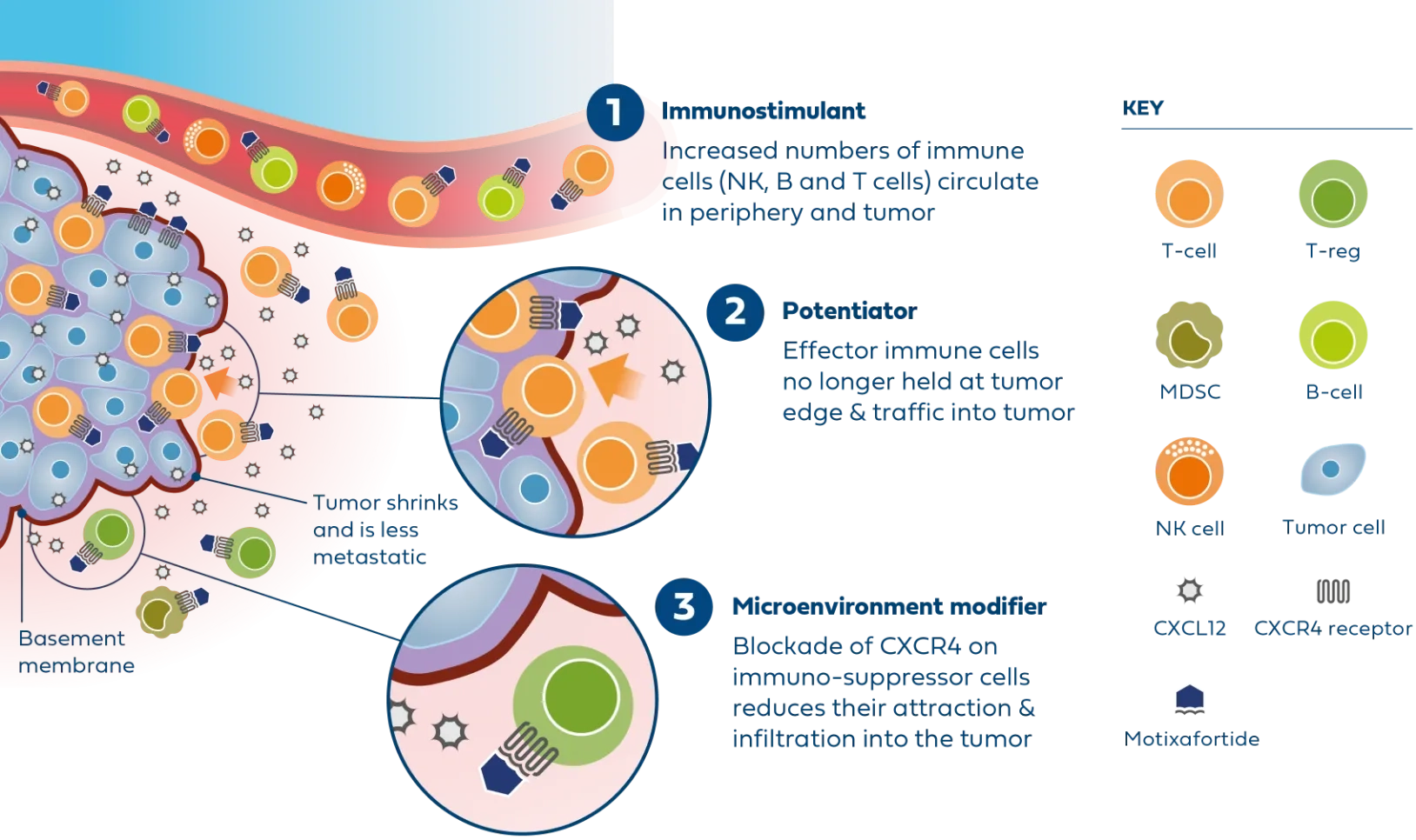
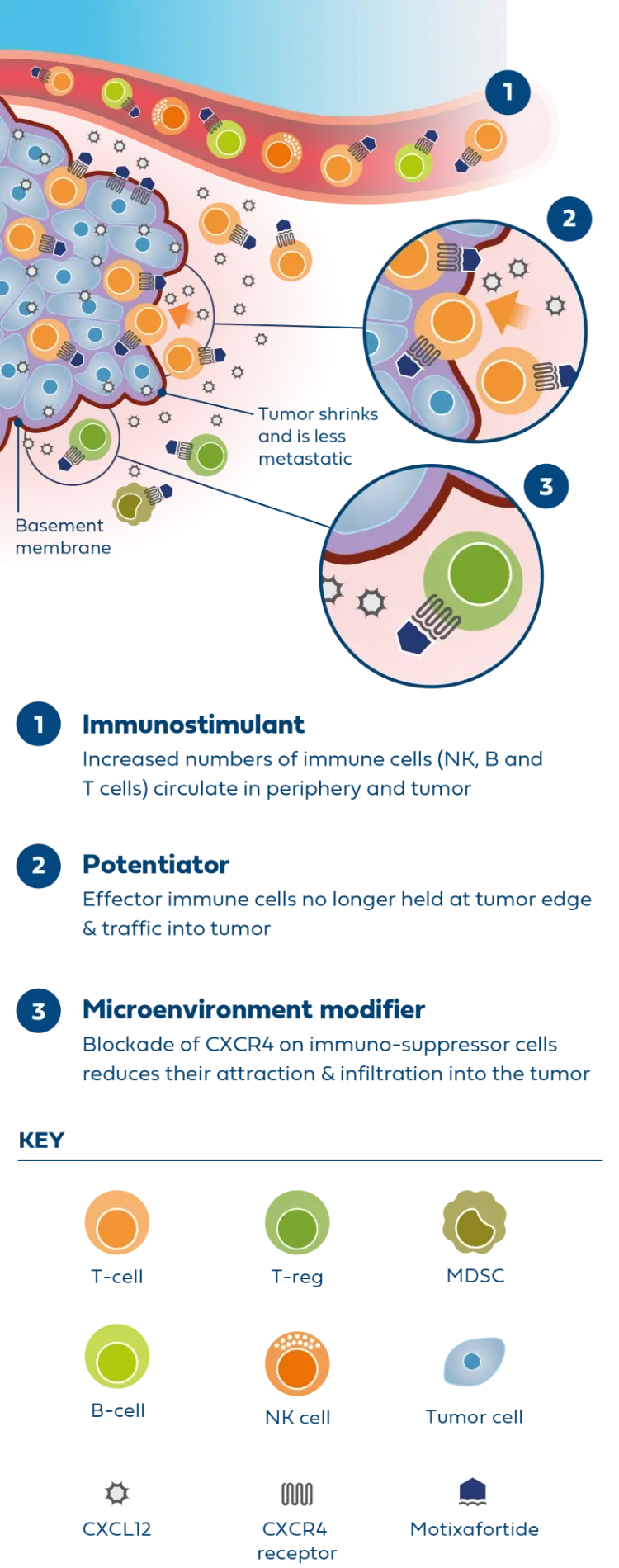
The safety and efficacy of the following investigational use of the marketed product motixafortide has not been established. The use has not been approved by the U.S. Food and Drug Administration or other regulatory authorities.
Pancreatic ductal adenocarcinoma (PDAC) is the most common type of pancreatic cancer.1 It is a highly aggressive cancer that is associated with poor patient outcomes because efficacious therapies do not yet exist.2
For all stages, the five-year survival rate is only 12 percent3 – the highest mortality rate in the U.S. among solid tumor malignancies. Globally, nearly a half million people were diagnosed in 2020 alone.4
Due to factors including an aging society,5 and increased rates of obesity and type 2 diabetes,6,7 PDAC incidence is growing. It is estimated that there will be 815,000 cases by 2040.8
Newer treatments like immunotherapy have limited efficacy, demonstrating a clear need to co-target alternative pathways.
COMBAT Phase 2a Study | Proof-of-Mechanism, Initial Safety and Efficacy in a Cohort of Patients with Second-Line Disease
Motixafortide is the most advanced CXCR4 antagonist in clinical development for metastatic PDAC and was successfully evaluated in the second part of the Phase 2 COMBAT study in combination with KEYTRUDA® (pembrolizumab) and chemotherapy (Onivyde plus 5-Fluorouracil/Leucovorin) as second-line treatment in patients with metastatic PDAC.
In patients with very advanced disease, results from the COMBAT study demonstrated improvements across all endpoints compared to available historical control data, including overall survival, progression-free survival, and overall response rate.
The triple combination was generally well tolerated, showing favorable safety and a low incidence of neutropenia and infections in treated patients.

6.5months
Median Overall Survival
compared to 4.7 months based on historical data9
Median Overall Survival
compared to 4.7 months based on historical data9

4months
Median Progression-Free Survival
compared to 2.7-3.1 months based on historical data10,11
Median Progression-Free Survival
compared to 2.7-3.1 months based on historical data10,11
The combination was well tolerated and showed a favorable safety profile
There was a low incidence of infections in treated patients
CheMo4METPANC Randomized Phase 2b Study In Patients with First-Line Disease
With proof-of-mechanism and concept established in the COMBAT Phase 2a clinical trial, motixafortide is now being studied in a large, randomized Phase 2b trial in partnership with Columbia University and supported equally by BioLineRx and Regeneron.
The multicenter trial is evaluating the combination motixafortide, PD-1 inhibitor LIBTAYO® (cemiplimab), and standard of care chemotherapies gemcitabine and nab-paclitaxel, vs gemcitabine and nab-paclitaxel alone in 108 patients. The study’s primary endpoint is progression-free survival, and secondary objectives include safety, response rate, disease control rate, duration of clinical benefit, and overall survival.
Preliminary efficacy data from the single-arm pilot phase of the CheMo4METPANC study with 11 patients was promising and prompted an amendment to the trial design to become a randomized study.
Pilot phase conclusions are summarized below (as of July 2023):
64%
Overall Response Rate12
Overall Response Rate12
9.6months
Median Progression-Free Survival12
Median Progression-Free Survival12
One patient experienced resolution of hepatic (liver) metastatic lesion12
The combination demonstrated a tolerable safety profile12
No unexpected Grade 4 or 5 treatment related adverse events12
Learn about active clinical trials: