The safety and efficacy of the following investigational use of the marketed product motixafortide has not been established. The use has not been approved by the U.S. Food and Drug Administration or other regulatory authorities.
Sickle cell disease is an inherited blood disorder, and one of the most common inherited genetic conditions in the world. In the U.S., the lifelong disease affects approximately 100,000 people.1
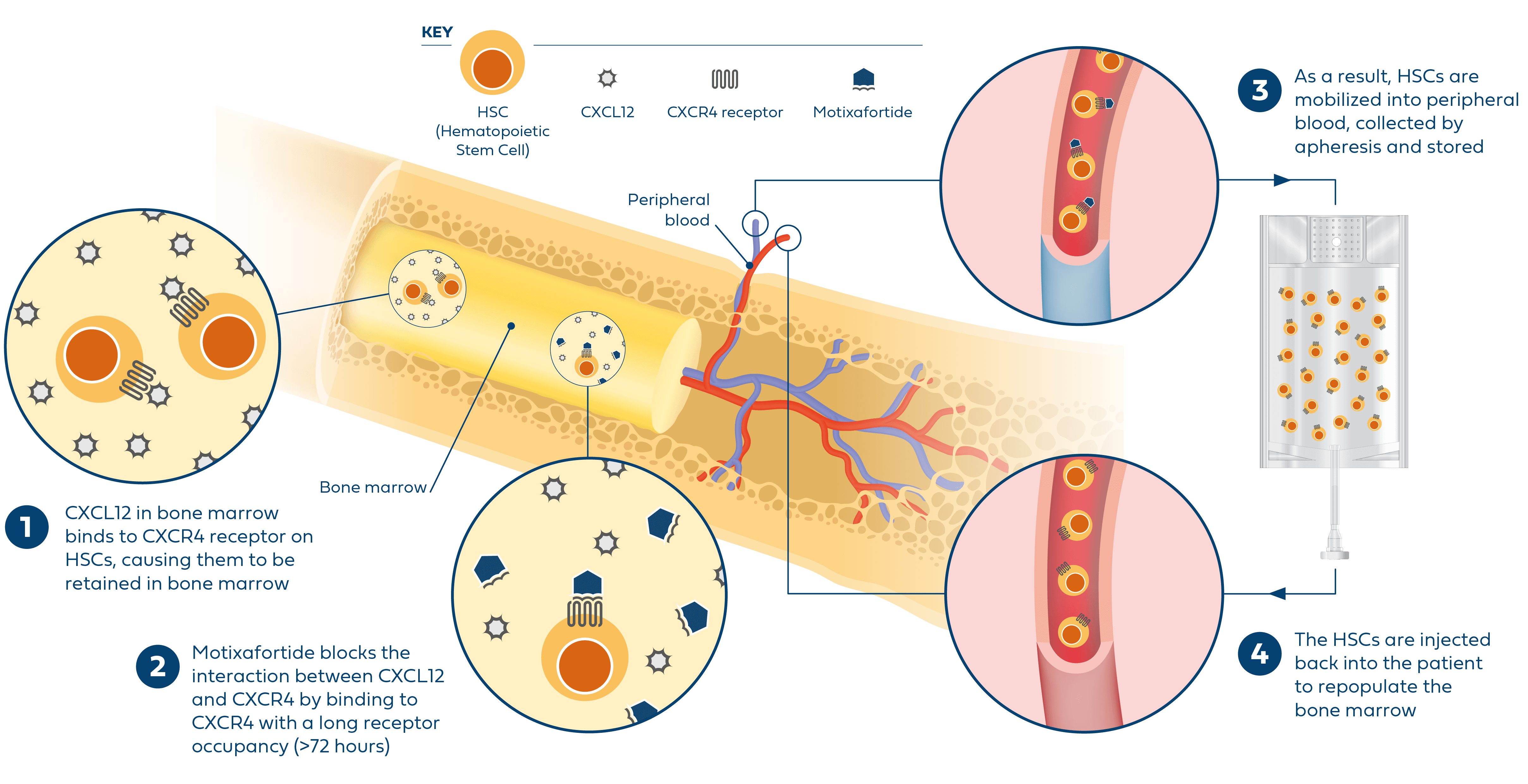
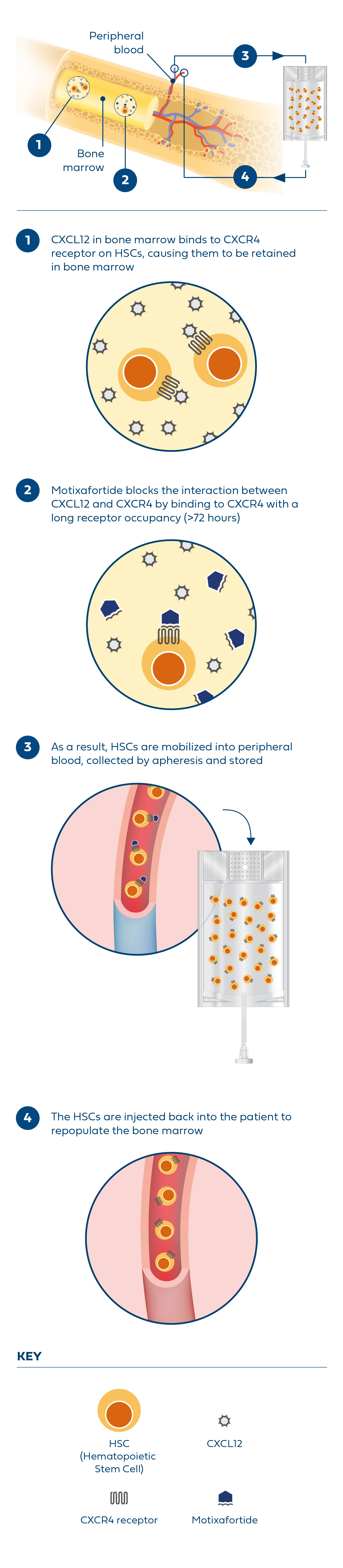
Hematopoietic stem cell (HSC)-based gene therapies are a promising field of medicine that focus on treating various diseases by targeting HSCs, which reside in the bone marrow and give rise to all blood cell lineages.2 Effective HSC-based gene therapies depend upon the collection of significant quantities of stem cells to engineer the treatments that enable the potential genetic treatment of various conditions, including but not limited to sickle cell disease. However, currently available mobilization regimens can carry serious risk and side effects for patients with sickle cell disease or may not reliably yield optimal numbers of HSCs for gene therapy.
Phase I Trials of Motixafortide to Mobilize HSCs for Sickle Cell Disease Gene Therapy
To potentially expand mobilization options, BioLineRx – in collaboration with Washington University School of Medicine – is advancing a Phase I clinical trial that is evaluating the safety and feasibility of motixafortide* as monotherapy and in combination with natalizumab (VLA-4 inhibitor) to mobilize HSCs for gene therapies in patients with sickle cell disease.
In a separate Phase 1 clinical trial sponsored by St. Jude Children’s Research Hospital, Inc., the safety, tolerability, and feasibility of single-agent motixafortide is being evaluated for the mobilization and collection of CD34+ HSCs in patients with sickle cell disease.